Steps to isolate the developmentally important player in our body – The Wnts
Doctora
The heart and soul of my doctoral thesis was related to the “Wnt” protein.Wnt signaling controls a variety of adult and developmental processes. The need to prevent the inappropriate activation of Wnt signaling is underscored by the fact that mis-regulation of Wnt signal transduction pathway leads to tumor formation. Thus, understanding the mechanisms that regulate Wnt signaling is of critical importance. In 2012, the year I joined Robert Ryan’s lab (or Bob, my PhD advisor and a lipoproteins and lipids wizard), coincided with the end of Bob’s sabbatical in Roel Nusse’s lab at Stanford (a bard in Wnt biology). Scientists that same year, had a breakthrough in the structure of Wnts after nearly 30 years. Wnt proteins are found in all multicellular organisms and as many as 19 mammalian homologs are known. To understand how Wnts influence cell signaling, one must measure its effects in a concentration-dependent manner. Wnt3a, one of the homologs has a fatty acid attachment making it hydrophobic and requiring detergent to stay in solution. However, the presence of detergent impedes biochemical assays. To address this pressing problem in the field, we reported an improved procedure for isolation of recombinant Wnt3a and introduced a novel strategy to eliminate detergent from isolated Wnt preparations. Our finding enables scientists to conduct studies that are closely relevant to the physiological microenvironment. In addition, using the improved protocol, significantly higher quantities of purified Wnt3a can be isolated.
Related publication:
Isolation and characterization of recombinant murine Wnt3a
Abstract of work presented at the Arteriosclerosis, Thrombosis and Vascular Biology/ Peripheral Vascular Disease conference in 2015
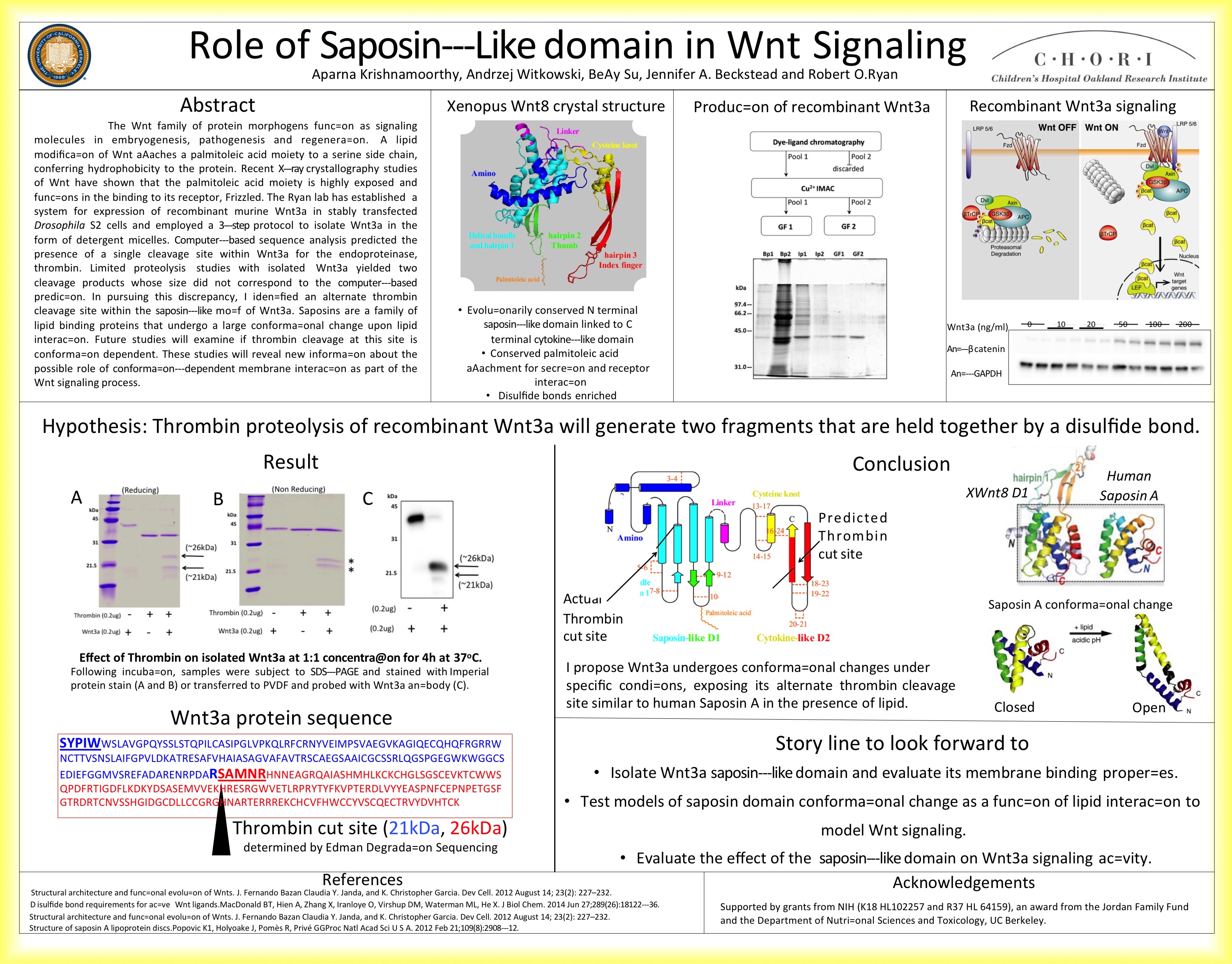
The Wnt family of protein morphogens function as signaling molecules in embryogenesis, pathogenesis and regeneration. A lipid modification of Wnt attaches a palmitoleic acid moiety to a serine side chain, conferring hydrophobicity to the protein. Recent X-ray crystallography studies of Wnt have shown that the palmitoleic acid moiety is highly exposed and functions in the binding to its receptor, Frizzled. The Ryan lab has established a system for expression of recombinant murine Wnt3a in stably transfected Drosophila S2 cells and employed a 3‐step protocol to isolate Wnt3a in the form of detergent micelles. Computer-based sequence analysis predicted the presence of a single cleavage site within Wnt3a for the endoproteinase, thrombin. Limited proteolysis studies with isolated Wnt3a yielded two cleavage products whose size did not correspond to the computer-based prediction. In pursuing this discrepancy, I identified an alternate thrombin cleavage site within the saposin‐like motif of Wnt3a. Saposins are a family of lipid binding proteins that undergo a large conformational change upon lipid interaction. Future studies will examine if thrombin cleavage at this site is conformation dependent. These studies will reveal new information about the possible role of conformation‐dependent membrane interaction as part of the Wnt signaling process.